4. Handling, Storage and Transport of Hydrogen
4.1 Physical and Chemical Conditioning of Hydrogen
Hydrogen still requires further preparation according to
the demands of the intended end use. Firstly, cleaning of the hydrogen is required in
order to ensure the required quality is met. Following this it must be compressed, whereby
the pressure level is dependent on either the end application or the intermediate storage
method. Alternatively, liquefaction may be the sensible option if transport over long
distances is required or if the end user require a high energy density (small storage
volume).
4.1.1 Cleaning
Depending on the type of impurities present and the
required final purity, different processes are applied. If the hydrogen is produced using
reforming, partial oxidization or pyrolysis processes, then any unwanted components can be
removed directly at the production stage. It is only the fine cleaning that is carried out
on the product hydrogen. Due the complex equipment required for chemical and physical
processes, they are usually only considered for large centralized plants, with catalytic
processes being more suited to decentralized applications. Their development into
inexpensive competitive products is still a long way from being complete and therefore it
is only possible at present to give an overview of the technical possibilities for
decentralized applications.
4.1.1.1 Cleaning of the feedstock
If the hydrogen is produced from oil, coal, natural gas or
biomass, using reforming or partial oxidization, then unwanted components of the feedstock
gas can be removed beforehand. Above all this is applied for dust removal in carbon gas or
biogas, the desulfurization of natural gas and the removal of CO2. The possible
presence of chlorine or heavy metal content (e.g. Mercury) in the raw gas can, as can
Sulfur, damage the catalysts of reforming plants and must be separated in a pre-cleaning
stage.
Present state of the art
- Dust removal:
Process description: Cyclone separators have only
limited application, as with this method only particles larger than 5 m m are removed
with a separation rate of 98%. For a fine separation appropriate filters must be used,
where, depending on throughput and purity demands, electrostatic filters (residual dust
content 75 - 100 mg/Nm3), „Schüttschichtfilter" (residual dust
content 50 mg/Nm3 ), mesh filters (residual dust content 10 mg/Nm3)
or cartridge filters (residual dust content <5 mg/Nm3) are used.
- Desulfurization:
Process description: A preliminary desulfurization
process is particularly necessary in the reforming of natural gas, in order to prevent
damage to (deactivation of) the nickel or platinum catalysts (Natural gas is odourized
with a sulfur containing substance). A whole series of chemical and physical processes
have been developed for this purpose, most of which are presently available as standard
for large reforming plants. The development of specialized methods for small decentralized
plants is still being pursued in earnest, with catalytic process appearing to be the most
relevant. In the field of natural gas cleaning there are three well proven processes,
known as the MEA-process, the MDEA-process and the Purisol-process. The first two
processes apply chemical absorption techniques using monoethylamine and methyldiethylamine
correspondingly. The Purisol method is a physical washing process in which COS compounds
are first converted into H2S and the solvent then selectively absorbs the H2S.
For the cleaning of biogas and other process gasses, the application of active carbon has
proved to be successful. Several processes have been developed in connection with the
gasification of coal which are based on an adsorption reaction followed by a chemical
reaction with metal oxides. Up to now, use of a ZnO cartridge (ZnO + H2S 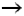 ZnS + H2O) for
treatment of natural gas with low sulfur concentration in small decentralized plants has
proved successful. This process is used for example in the 200 kWel-PAFC
cogeneration units from ONSI for natural gas treatment. The sulfur content of the
feedstock must however not exceed 1%. The removal of 1 g of sulfur requires
2.4 g of ZnO such that according to ONSI, a single cartridge is sufficient for 5
years operation of a PC-25 fuel cell rated at 200 kWel and with a sulfur
content reduction from 6 ppm down to less than 1 ppm.
ZnS + H2O) for
treatment of natural gas with low sulfur concentration in small decentralized plants has
proved successful. This process is used for example in the 200 kWel-PAFC
cogeneration units from ONSI for natural gas treatment. The sulfur content of the
feedstock must however not exceed 1%. The removal of 1 g of sulfur requires
2.4 g of ZnO such that according to ONSI, a single cartridge is sufficient for 5
years operation of a PC-25 fuel cell rated at 200 kWel and with a sulfur
content reduction from 6 ppm down to less than 1 ppm.
- CO2-washing
Process description: Chemical absorption processes
exploit the chemical reaction between a contaminant and a washing solution. In the
regeneration stage the contaminant can be forced back out of the washing solution (by the
use of different temperature levels). The processes are best suited for the removal of
acidic components of the gas. This is therefore the preferred method for H2S,
COS, HCN and CO2 removal.
Who offers cleaning processes? Depending on the size
of a reformer or partial oxidizer, preliminary cleaning equipment is usually offered as
part of the entire plant. Likely suppliers are therefore the constructors of chemical
plants like Linde, Uhde, Höchst, Lurgi, and particularly for desulfurization,
Galvanoform.
How much does hydrogen cleaning cost? The costs of
gas pre-cleaning vary strongly depending on the individual demands for plant size and
required purity as well as depending on the level of contamination. It is therefore
impossible to give specific prices here.
What is presently under development?
One of the major areas of present development is that of
small decentralized cleaning plants. This is due to the expectation of large markets for
decentralized fuel cell applications. In particular, the ZnO process which is already in
use is being further developed.
4.1.1.2 Post production cleaning processes:
In a post production cleaning stage, all remaining unwanted
elements are separated from the hydrogen gas. This particularly applies to the products of
incomplete reforming like CO, H2O, O2, NH3 and CO2.
The catalytic processes described below serve exclusively for the removal of CO, while the
other processes discussed can also be applied for other elements depending on the
materials used.
Present state of the art
- Catalytic Process
For catalytic processes like CO conversion (CO + H2O
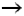 CO2 + H2),
selective methanisation (CO + 3 H2
CO2 + H2),
selective methanisation (CO + 3 H2 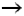 CH4 + H2O) or selective CO oxidation (CO +
½ O2
CH4 + H2O) or selective CO oxidation (CO +
½ O2 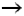 CO2)
the achievable efficiencies are dependent on reaction parameters such as temperature,
pressure, volumetric flow, raw gas concentration, and catalyst material. Normally,
different processes are combined together, with impurity levels of 1 % to only a few
ppm possible depending on the exact procedure used. The selection of reaction paths is
dependent on the use of different catalyst materials.
CO2)
the achievable efficiencies are dependent on reaction parameters such as temperature,
pressure, volumetric flow, raw gas concentration, and catalyst material. Normally,
different processes are combined together, with impurity levels of 1 % to only a few
ppm possible depending on the exact procedure used. The selection of reaction paths is
dependent on the use of different catalyst materials.
- Adsorption Process
The highest purity of up to 99.999% H2 content
is obtained using the pressure swing adsorption (PSA) method. In this process the raw
hydrogen is forced under pressure through an active carbon filter or a carbon-molecular
sieve. The process is discontinuous because regeneration of the filter by cleaning is
required at regular intervals. The related but seldom used temperature swing adsorption
process (TSA) is also suitable for the removal of CO2, H2S, COS, H2O,
O2, NH3 and Hg.
- Membrane process
Membrane processes exploit the selective transmission
characteristics of the membrane material for different molecules, whereby the most
effective membranes are also the most expensive (Palladium membrane). These membranes are
nowadays used to some extent for highest purity in the chemical and microelectronics
industries. A small purifier with Ag/Pd membrane and throughput of 0.3 Nm3/h
has been developed by CJB-Development Limited.
- Metal Hydride
Gas purifiers based on metal alloys are mainly used for the
production of the purest gasses for the semiconductor industry. A raw gas pre-cleaning
using other methods is necessary allowing for a further cleaning with metal hydrides,
which raises purity levels by two orders of magnitude.
Who can supply cleaning plants?
Large centralized cleaning plants like the catalytic and
adsorption processes are offered as part of total reformer packages by large plant
engineering companies.
Membranes are commercially available by large plant
engineering companies like Dow Chemical and Hoechst. Small decentralized units with
throughputs from 0,3 Nm3/h are available from CJB-Development Limited. Advanced
filters, manufactured using palladium foil on a supporting substrate can be obtained from
Bend Research.
The main German supplier of metal hydrides for cleaning
purposes is GfE (Gesellschaft für Elektrometallurgie). This company is also carrying out
further development in this area.
What is the price of gas cleaning equipment?
The costs of catalytic processes are usually included in
the total price of a reformer. A pressure swing adsorption device is an add-on and costs
about 1 million DM for one with 1000 - 1500 Nm3/h capacity. Membrane and metal
hydride cleaners are usually very expensive due to the high costs associated with the
special materials involved in obtaining a large active area. The prices are very dependent
on the specific demands concerning cleaning capacity, and initial gas quality. For
example, presently available small palladium filters have a worldwide price variation in
the range from 300 DM up to 5000 DM per Nm3/h throughput.
What is presently under development ?
The most promising market for purifiers is that of small
purifiers for decentralized applications. Correspondingly, the major development work is
focusing on this area, particularly on the testing of less expensive materials for the
membrane and metal hydride processes. In this respect, a porous ceramic membrane has been
successfully used in Israel (Weizmann Institute) for the separation of CO from hydrogen.
Experiments have also been carried out recently with semi-permeable polysulfone membranes
since these seem to offer large possible cost savings. Considerable cost reductions are
likewise being sought for the expensive palladium filter technique. This is being
attempted via specialized doping and above all the use of a cheaper substrate with a very
thin active layer.
A short time ago, a metal hydride (on Ni-Al base) was
successfully used for the first time for the raw gas conversion. This seems to be
promising due to the fact that cleaning stage can be carried out by a storage medium
itself and thus reduce overall costs. A similar process (cleaning and storage with the
same material) can be achieved with iron oxide storage and this system has large potential
for cost reductions. Experimental work in this area has been, and continues to be carried
out mainly by H-Power (USA), at the Technical University Graz (Austria) and at the Center
for Solar Energy and Hydrogen Research (ZSW) in Ulm (Germany).
4.1.2 Compression
Depending on the desired use, hydrogen must be either
compressed or liquefied. Compression of hydrogen is carried out in the same way as for
natural gas. It is sometimes even possible to use the same compressors, as long as the
appropriate gaskets (e.g. Teflon) are used and provided the compressed gas can be
guaranteed to be oil free.
The work required for compression, wt, ith, for
isothermal compression (cooled chamber compression) is calculated according to :
wt, ith = RH2 T Z ln
(p2/p1)
Where:
| RH2 = |
4124 J/kgK (hydrogen gas constant) |
| T = |
Temperature |
| Z = |
(K(p1) + K(p2)))/2K(p1)
(Correction factor for hydrogen gas with K(p) = 1 + p/150 MPa) |
| p2 = |
high pressure level |
| p1 = |
initial pressure |
To calculate the required motor capacity PM, the
mass throughput and the compression efficiency have to be considered.:
PM = m/t 1/h
Where:
| m/t = |
mass throughput per unit time, |
| h = |
efficiency considering all hydraulic and
mechanical losses (this figure is about 50% for small plants) |
This logarithmic relationship between the work required and
the compression level shows clearly that the initial pressure dominates the level of work
required for compression. For example, a compression from 1 to 10 bar requires about the
same energy input as a compression from 10 to 100 bar. Along with the investment costs,
this is particularly noticeable in the operating costs (gas or electricity consumption).
Usually this kind of compression is carried out in multiple stages with the first stage
providing a pre-pressurization into the several atmospheres range.
This first stage can be avoided if the hydrogen is produced
via a high pressure electrolyser. For a cost optimized layout, the extra costs of the high
pressure electrolyser should be offset by the saved investment and operating costs of the
first compression stage.
Present state of the art
Since hydrogen compression is carried out in the same way
as compression of natural gas, the procedure is well tested and readily available. New
developments are mainly associated with the optimization of the individual units within
the total concept, with the primary application here being the high pressure compression
at service stations. Typical pressure levels are 3 - 4 MPa for pre-compression stages for
filling of collecting tanks, and 25 - 30 MPa for storage tanks in fast fill applications.
The fast fill process is achieved by an over pressure over the pressure level in the
vehicle tank being filled (20 or even 25 MPa). The choice of the highest pressure level is
primarily dependent on the maximum permitted pressure that the storage tank can withstand
(modern tanks constructed from composite materials are rated for up to 30 MPa). Because of
the logarithmic relationship between pressure and work required for compression, the
increased energy required for a higher filling pressure is not that great. Thus the
compression from 0.1 to 30 MPa needs only 10% more energy than the compression from 0.1 to
20 MPa.
Almost all common natural gas compressors can be easily
modified to be suitable for hydrogen. The range of available compressors for hydrogen
therefore ranges from small units with several Nm3/h throughput up to those
with several hundred Nm3/h. Compressors are primarily used for the filling of
stationary high pressure (20 - 30 MPa) and low pressure (1 - 5 MPa) storage tanks. The
planning of the highest pressure level can only be determined in connection within the
context of the application.
Unlike the natural gas case, large compressors in the MW
range used for long distance transport application are not being optimized or used for
hydrogen. The reason being that no such demand is predicted in the foreseeable future.
Who supplies compressors?
Presently, the firms supplying natural gas compressors also
have a range of hydrogen models available. Two firms particularly worth mentioning are
Sulzer-Burkhardt and in Germany, Mannesmann DEMAG.
What does a compressor cost?
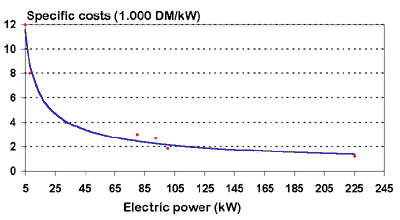
The exact costs are determined by the choice of a
compressor correctly dimensioned in the context of the entire system. As a rough guide,
the figure below shows capacity dependent cost data. This graph has been put together
according to actual specific offers where it is assumed that the capacity specific price
is approximately independent of the application parameters (throughput, pressure
difference). Accordingly, prices for small plants (around 10 kWel) can be
expected to be up to 10,000 DM/kWel, with prices falling with increasing plant
size to the point where a 250 kWel unit will cost around 1000 - 1500 DM/kWel.
4.1.3 Liquefaction
Process description: Liquefaction plants operate
nowadays with a liquid nitrogen pre-cooling of the feed hydrogen. This hydrogen supply
must have a pressure of at least 2 MPa. If the hydrogen is produced locally using a high
pressure electrolyser, then this eliminates the need for a preliminary compression stage.
Before being liquefied, the hydrogen must be cleaned for removal of CO2, CO, CH4
and H2O. This is usually done using a pressure swing adsorption process ( hydrogen cleaning
{Sprungmarkenach 4.1.1}(?)). Depending on throughput, various different liquefaction
process can be applied, with large plants usually operating with a combination of these
processes (turbine, Joule-Thomson, and magnetocaloric processes). In all cases the
liquefaction is achieved by compression followed by some form of expansion, either
irreversible via use of a throttle valve or partly reversible via the use of a expansion
machine. There are usually 6 heat exchanger stages with the first being cooled with liquid
nitrogen. Before the final stage, a Joule-Thomson valve takes care of the expansion. Using
magnetocaloric processes, a transformation from ortho-hydrogen to para-hydrogen is being
achieved. Over several stages, a para-hydrogen content of 95% is reached. Para-hydrogen,
with its symmetrical wave function has a lower energy content than ortho-hydrogen. In this
way, liquid hydrogen temperatures of 21 K are achieved.
hydrogen cleaning
{Sprungmarkenach 4.1.1}(?)). Depending on throughput, various different liquefaction
process can be applied, with large plants usually operating with a combination of these
processes (turbine, Joule-Thomson, and magnetocaloric processes). In all cases the
liquefaction is achieved by compression followed by some form of expansion, either
irreversible via use of a throttle valve or partly reversible via the use of a expansion
machine. There are usually 6 heat exchanger stages with the first being cooled with liquid
nitrogen. Before the final stage, a Joule-Thomson valve takes care of the expansion. Using
magnetocaloric processes, a transformation from ortho-hydrogen to para-hydrogen is being
achieved. Over several stages, a para-hydrogen content of 95% is reached. Para-hydrogen,
with its symmetrical wave function has a lower energy content than ortho-hydrogen. In this
way, liquid hydrogen temperatures of 21 K are achieved.
Present state of the art
There are presently several small liquefiers with daily
output of 200 kg in service mainly for research purposes. In the USA, construction of
the first five large scale industrial liquefaction plants began in the mid fifties. These
plants had capacities between 15,000 - 35,000 l/h (approx. 25 - 60 t/d). At about the same
time, Linde AG installed a plant in India with 10,000 l/h (16 t/d) capacity.
Today there are about 10 medium sized plants with production capacities of 10 - 60 t/d, in
operation around the world. Newer are liquefaction plants in USA, Japan and Europe with
capacities in the range 2000 to 8000 l/h (3 - 12 t/d). 1991 saw the construction and
commissioning of a liquefaction plant in Ingolstadt, Germany. This plant supplied by Linde
AG, has a capacity of 4.4 t/d.
Who sells liquefaction plants ?
The two big manufacturers presently offering hydrogen
liquefiers are Air Liquide and Linde AG. Except for research plants, the smallest
liquefaction plant with somewhat more than 1 t/d capacity, has been constructed in India.
What is the cost of a liquefaction plant ?
A liquefaction plant with 4.5 t/d capacity costs around 30
Million DM. Since the costs are heavily dependent on material usage, the costs increase
with capacity according to
I1/I2 = (K1/K2)0,6...0,7,
where I are investment costs and K represents the plant
capacity. The total costs are made up of planning (10%), components (60%) and construction
and erection (30%). A construction time of 2.5 to 3 years is to be expected.
In terms of fuels, for each liter of LH2 (this
corresponds to 2.36 kWh or 210 g hydrogen) about 0.9 kWel, 45 l
cooling water and small amounts of nitrogen are required. Along with personnel costs, this
leads to yearly operation and maintenance costs in the order of 2.5% of the original
investment.
Small liquefaction plants are, in comparison, considerably
more expensive. A drastic price reduction would be possible in this area if standardized
production with high product numbers was to be carried out. Presently however such a
situation is not foreseeable.
What is presently under development?
In the coming years sinking investment costs are to be
expected, with a reduction of up to 25% considered possible while maintaining present
energy efficiency. A reduction in the energy requirements is expected in the medium term
with the consumption probably falling to 0.8 kWhel/lLH2. In the long
term, further developments in the magnetocaloric process could improve the energy
efficiency considerably more. Values as low as 0.35 kWhel/lLH2 are
considered achievable for commercial plants.
4.2 Storage possibilities applicable
for Hydrogen
In this chapter a comparison between hydrogen and natural
gas storage will be carried out considering the volume, weight, arrangement and type of
possible storage devices.
4.2.1 Compressed gas storage
4.2.1.1 Stationary Storage
Present state of the art
- Stationary large scale storage
In principle, the storage techniques used for natural gas
are also applicable for the compressed storage of hydrogen. For large seasonal storage
applications, underground porous storages, aquifers, salt caverns or rock caverns are used
because it offers the most inexpensive solution. England and France both have long term
experience in the field of underground hydrogen storage. The British chemical concern ICI
stores hydrogen in three brine compensated salt caverns in Teeside, England. The hydrogen
is stored at pressures up to 50 bar in these up to 366 m deep caverns. From 1957 until
1974, GAZ DE FRANCE stored towngas with a 50% hydrogen content without problem in a 330
Mio. m3 aquifer storage. This underground hydrogen storage method is about two
orders of magnitude cheaper than tank storage. It is, however, only relevant for volumes
of several million Nm3.
- Stationary small scale storage
In the natural gas sector, buffering of short term
fluctuations due to pressure variations in the distribution network can be achieved using
disk or bell gas storage or low pressure spherical tanks (1.4 MPa, 15,000 m3).
Operation experience of this kind is not yet available for the case of hydrogen.
Small stationary storage is carried out without exception
in the form of above ground compressed gas devices. In the industrial sector a
standardization of type has already occurred. As a result, cylindrical tanks with a
maximum operating pressure of 5 MPa and 2.8m diameter are now available in the following
lengths (or heights) : 7.3 m (max. capacity at 4.5 MPa: 1305 Nm3), 10.8 m (max.
capacity 2250 Nm3) and 19 m (max. capacity 4500 Nm3). In these
cases, calculations for energy density by weight and volume including the storage device
itself result in figures of 0.24 - 0.31 kWh/kg and 0,135 kWh/l, respectively.
Bottle type storage can also be used as stationary storage
as long as the volume is sufficient. Such bottles are available in steel in sizes ranging
from 2 to 50 l (corresponding to 0.35 - 8.9 Nm3 and weights of 5.3 - 68 kg)
with operating pressures of 20 MPa.
Who offers small stationary storage devices
As a rule these devices are available from manufacturers of
technical gases.
How much do they cost
These storage devices are often not bought but rather
rented. Monthly rental charges of several thousand DM and also a one-off initial cost of
several thousand DM are to be expected. These prices are of course dependent on the size
of storage required. It is then worth making a purchase when the period of usage is going
to extend over several years. Corresponding, Rent/Buy comparisons should be carried out on
an individual basis.
4.2.1.2 Mobile Storage
Present state of the art
In the last few years, the introduction of natural gas
driven automobiles has seen the development of compact mobile pressurized gas tanks. These
tanks are generally rated for filling pressures of 20 MPa. As a result of the American
practice of increasing storage pressure and hence storage density, there are now some
tanks available with rated filling pressures of 24.8 MPa or even 30 MPa suitable for
hydrogen as well as natural gas. Because of the weight advantage, the last few years has
seen the replacement of steel tanks with composite tanks (full-composite, alu-composite)
in the mobile area. These tanks have sizes ranging from 50 l (Length: 953 mm, Diameter:
310 mm) to 392 l (Length: 6000 mm, Diameter: 335 mm).
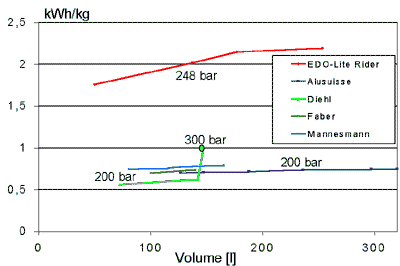
An overview of presently available mobile hydrogen tanks is
given in the figure below. The lowest storage density, at 0.5 kWh/kg, is achieved with
steel tanks and 20 MPa pressure. The highest storage density is that obtained with
lightweight full-composite bottles which have rated operating pressures in Germany of 24.8
MPa.
Who offer mobile storage devices.
In Germany, mobile hydrogen storage devices are available
from MAN, ALUSUISSE, Diehl, Faber und Mannesmann.
How much do these devices cost.
The costs are very dependent on the number of devices
purchased. As an example, a modern full-composite bottle with 150 l volume is
presently offered for about 8000 DM.
What is presently under development
In the last few years the development has concentrated on
weight reduction via the use of modern composite materials and at the same time increasing
capacity through higher pressure levels. It is also expected that this development will
continue for some time. The present costs are dictated by the very low production
quantities which means that considerable cost reductions would be possible if the demand
rises accordingly.
4.2.2 Liquid gas storage
4.2.2.1Stationary storage
Present state of the art
The storage technology for liquid hydrogen is presently
state of the art thanks to extensive application in space travel. This form of storage is
usually carried out with storage tanks having perlit vacuum insulation. There are many
such tanks in the USA, the largest of which belongs to NASA and is located at Cape
Canaveral. This tank has a storage volume of about 3800 m3 (approx. 270 t LH2).
With an outer spherical diameter of 20 m, the evaporation rate is under 0.03% per
day, allowing for a storage period of several years. As compared with pressurized gas
storage, this method offers more inexpensive storage costs when dealing with large
quantities.
The storage of liquid hydrogen on a small scale, i.e.
transportable containers up to 100 l capacity, is presently state of the art just as the
technology for liquid helium. Larger containers are to some extent produced with perlit
vacuum insulation, while smaller storage devices rather tend to apply super insulation and
continuous cold gas cooling (shielding). Vacuum super isolated tanks can achieve
evaporation rates in the order of 0.4% per day. Large tanks with vacuum powder insulation
have rates in the range 1% - 2% per day, depending on geometry.
Common stationary tanks have capacities ranging from 1500 l
(approx. 1100 Nm3 ) up to 75,000 l (approx. 60,000 Nm3) with radii
of 1400 mm to 3800 mm and heights of 3060 mm up to 13,977 mm.
Who offers liquid gas tanks
Liquid hydrogen tanks are available from suppliers of
technical gasses, (Linde, Air Liquide, Messer-Griesheim).
How much do liquid hydrogen tanks cost
Because of the high cost of insulation, liquid hydrogen
storage is expensive. Despite the high price however, in the case of large tanks the
increased storage density of liquid hydrogen outweighs the benefit of reduced material
costs associated with compressed gas storage.
What is presently under development
The focus of present development is the search for less
expensive insulation material and production methods.
4.2.2.2 Mobile Storage
Present state of the art
In association with activities regarding hydrogen vehicles,
small mobile storage devices have been developed in Germany. Tanks for cars (installed in
BMW’s test vehicle) and busses (installed in the MAN-Bus SL 202) are presently
available as individually manufactured items. The bus tank actually consists of three
elliptical cross section tanks each with 190 l capacity, corresponding to an energy
content of 450 kWh or 150 Nm3. Energy densities of 4.5 kWh/kg or 2.13 kWh/l are
achieved. The tanks are constructed from 200 - 300 layers of insulating foil, enabling
evaporation rates of around 1% per day to be achieved. This figure is increased however
when multiple tanks are connected together, due to losses in the connecting pipes.
Who offers mobile liquid gas tanks
Mobile liquid hydrogen tanks were developed by and are
available from Messer-Griesheim, Linde AG and Air Liquide.
How much do mobile liquid hydrogen tanks cost
Since the presently available tanks are all one-off
productions, their prices are not representative. For further price projections, concrete
offers need to be obtained from the manufactures according to specific production
quantities.
4.2.3 Metal hydride Storage
Present state of the art
Within the framework of research activities at Daimler-Benz
in the eighties regarding hydrogen cars, a daughter firm was founded in cooperation with
Mannesman with the intention of optimizing the technology of metal hydride storage.
Nowadays these activities are carried out by GfE (Gesellschaft für Elektrometallurgie).
Advantageous of metal hydride storage is the low loading pressure (0.25 - 10 MPa depending
on material choice) while at the same time giving a high volumetric storage density
(approx. 0.21 - 0.39 kWh/kg and 1 - 1.5 kWh/l). The high weight of a metal hydride storage
device is the disadvantage. Because of the chemical properties, the loading of a metal
hydride storage device is accompanied by generation of heat. On the other hand, in order
to release the store hydrogen , heat must be applied. Depending on individual
applications, the desired pressure and temperature levels can be specified by the choice
of an appropriate alloy. It should however be realized that this process can lead to
reductions in the storage density. An optimized system presently has a maximum storage
capacity amounting to about 1.8% of the weight of the device that can be stored in the
form of hydrogen.
Since metal hydrides also demonstrate excellent cleaning
properties, both properties can be combined in certain applications.
Who offers metal hydride storage devices ?
In Germany, metal hydride storage is being further
developed and supplied by GfE. In the international scene it is the activities in Japan
and Canada that are most worth mentioning.
What is the cost of metal hydride storage
The cost of metal hydride storage is mainly dependent on
the desired properties (temperature level, pressure level, storage density, storage size).
As a guide, prices for a 30 Nm3-storage device (90 kWh) range between 13000 and
50.000 DM (with weights of 230 - 420kg and volumes 60 - 90 l). As a rough guide for price
scaling, manufactures give the following figures: 800 - 3000 DM for 1 Nm3 total
storage, 400 - 1500 DM/Nm3 for 10 Nm3 total storage, and 300 - 1100
DM/Nm3 for 100 Nm3 total storage.
What is presently being developed
The further development of metal hydride storage is
concentrated on increasing the storage capacity, with the intended goal being a doubling
of capacity. The reduction of costs is also being targeted. Should these goals be
achieved, then metal hydride storage could represent an interesting storage option for
mobile applications. The special advantage of metal hydride in this case is the technical
safety properties that make metal hydride a problem-free storage material.
4.2.4 General
Several other possibilities for hydrogen storage are
presently under development.
In America and Europe the development of a market
competitive iron oxide storage method (iron sponge) is being particularly intensively
pursued. As with the metal hydride storage, the storage and cleaning properties can be
combined. It is expected that the iron oxide storage method will offer huge advantages in
energy density and storage costs. A market competitive product is expected within the next
three to five years.
Along with this, another area being developed, particularly
in America, is the field of adsorption storage. This method would also offer advantages in
the area of storage density. Latest news on hydrogen storage using carbon micro fibers
indicate possibilities of achieving storage densities two orders of magnitude higher than
achieved so far, even going beyond the storage density of liquid hydrogen tanks. Should
these results of basic research be confirmed and should this technology prove to be
technically and economically feasible then this would represent a real breakthrough in
hydrogen storage. Also being investigated is the high pressure storage in so-called
„microspheres". Small glass spheres with diameters less than 100 microns can
withstand pressures up to 1000 MPa. This would then allow high storage densities to be
achieved in such a storage device. Since the permeation of hydrogen through the glass is
temperature dependent, the flow of hydrogen could be controlled by applying heat to the
storage device. The investigation of such a storage possibility is however only just
beginning.
4.3 Transport of Hydrogen
4.3.1 Transport of CGH2
Compressed hydrogen is these days delivered from producer
to consumer in mobile compressed tanks using trucks or trains. Alternatively it can be
supplied via a pipeline distribution network to which multiple suppliers and consumers are
attached.
Road transport is carried out using trucks carrying steel
bottles at 20 MPa with each vehicle carrying about 2400 - 3600 Nm3.
In Germany there are two large hydrogen distribution
networks: One in the Ruhrgebiet that is operated by BOC Gases under contract for Hüls AG
and one in the industrial area Leuna-Bitterfeld-Wolfen operated by Linde. Both networks
have over 50 km of pipeline and have been operating without major problem for over 50
years with pressures of 2 MPa. There are also several smaller hydrogen pipelines of
other firms in service. As well as for internal use these pipelines usually serve to
connect nearby producers (usually from the chlorine industry) and consumers. There is a
wealth of operating experience in connection with hydrogen. The hydrogen network in the
Ruhrgebiet is supplied by multiple producers (also mainly from the chlorine industry).
Linde’s hydrogen network is predominantly supplied by a single natural gas reformer
with 35000 Nm3/h capacity (corresponding to a yearly capacity of 280 Million Nm3/a)
as well as chemical producers from the Bitterfeld region.
Who offers hydrogen ?
Hydrogen is offered by industrial gases companies. Local
producers from the chemical industry also to some extent promote their own brand of
hydrogen. This is however only feasible within certain limited conditions, as the
corresponding conditioning costs of the hydrogen have to be taken into account.
What is the cost of Hydrogen
The transport of hydrogen is associated with costs that are
negotiated on a case by case basis with the corresponding delivery firm. An estimate of
present and future hydrogen costs will be dealt with in a chapter of its own. ( Costs of hydrogen).
Costs of hydrogen).
4.3.2 Transport of LH2
Liquid hydrogen is presently dealt with predominantly in
small quantities. In this respect road transport is carried out using trucks with about 50,000 l
capacity. Delivery is achieved either in vacuum insulated bottles or by refilling of
stationary tanks ( Hydrogen storage), depending on required quantities. In the
USA there are several pipelines for liquid hydrogen with lengths of up to 40 km.
Hydrogen storage), depending on required quantities. In the
USA there are several pipelines for liquid hydrogen with lengths of up to 40 km.
The intercontinental transport of hydrogen will be carried
out in liquid form using ships. For this purpose, specialized ships with appropriate tanks
and port facilities are being designed. A realization of these ideas will however not take
place until the trade in hydrogen reaches an appropriately large scale.
As an already market competitive option, the transport of
hydrogen in 40 foot containers is being prepared by Hydro-Québec. Since the production
and liquefaction of hydrogen in Québec is particularly inexpensive due to the low
electricity costs, this could lead to the availability of very inexpensive hydrogen within
the next two years.
Who deals with liquid hydrogen
In Germany, liquid hydrogen is produced and marketed
without exception by Linde in Ingolstadt. The hydrogen is produced as a by-product of
mineral oil industry. Other providers of liquid hydrogen like Air Liquide, Air Products or
Messer-Griesheim obtain the hydrogen outside of Germany (e.g. Rotterdam, Linz). As well as
these firms, there are also intermediate traders active in this area (e.g. AGA-Gas).
The marketing of imported liquid hydrogen from Canada using
container transport is in preparation. Up to now, no suppliers can be named.
What is the cost of liquid hydrogen
The costs for liquid hydrogen are very dependent on the
case specific factors such as delivery quantities as well as transport distance and
frequency. Prices therefore have to be negotiated on a case by case basis with the
supplier (see also Costs of hydrogen).
back to Contents
![]() ZnS + H2O) for
treatment of natural gas with low sulfur concentration in small decentralized plants has
proved successful. This process is used for example in the 200 kWel-PAFC
cogeneration units from ONSI for natural gas treatment. The sulfur content of the
feedstock must however not exceed 1%. The removal of 1 g of sulfur requires
2.4 g of ZnO such that according to ONSI, a single cartridge is sufficient for 5
years operation of a PC-25 fuel cell rated at 200 kWel and with a sulfur
content reduction from 6 ppm down to less than 1 ppm.
ZnS + H2O) for
treatment of natural gas with low sulfur concentration in small decentralized plants has
proved successful. This process is used for example in the 200 kWel-PAFC
cogeneration units from ONSI for natural gas treatment. The sulfur content of the
feedstock must however not exceed 1%. The removal of 1 g of sulfur requires
2.4 g of ZnO such that according to ONSI, a single cartridge is sufficient for 5
years operation of a PC-25 fuel cell rated at 200 kWel and with a sulfur
content reduction from 6 ppm down to less than 1 ppm.
