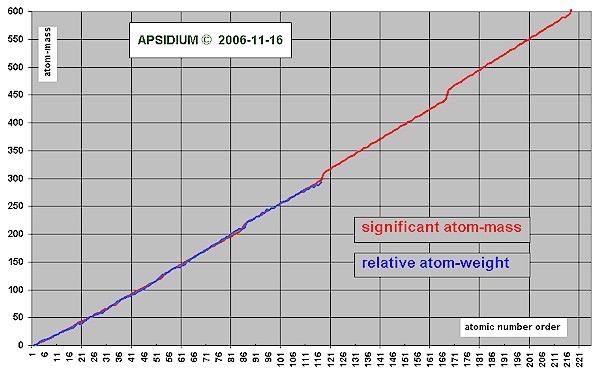
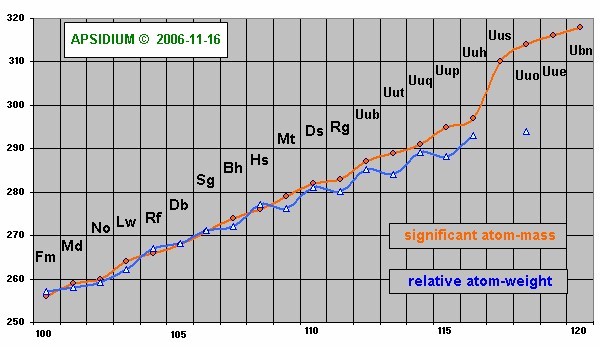
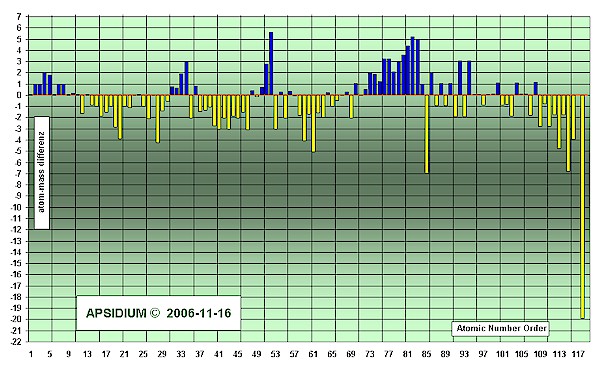
Is the significant atom-mass equivalent with the relative atom-weight?
No, usually both values are near with each other. The relative atom-weight calculates itself from the procentage of all isotopes of one element. The significante atom-mass is declared as completely-number (mostly in the middle of the isotopes). It gives a reference number with the known isotopes [Naturally Abundant] and with the unknown elements a trend to the likely possible and stable elements.
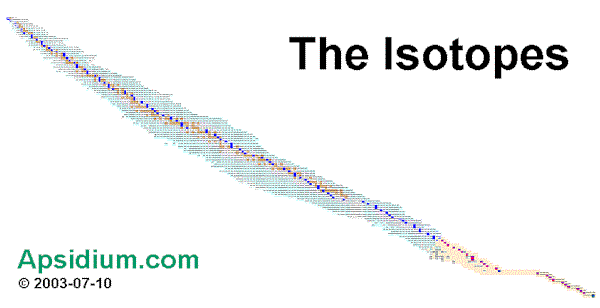
You can see such isotopes at the isotopes table. Attention, very long loading time! ![]() The elements with significant atom-mass are marked as elements with blue or red fields and white ink. [ see also: Moller Theoretical Nuclear Chat
The elements with significant atom-mass are marked as elements with blue or red fields and white ink. [ see also: Moller Theoretical Nuclear Chat ![]() ]
]