Meta-analysis
From Wikipedia, the free encyclopedia
In statistics, a meta-analysis combines the results of several studies that address a set of related research hypotheses. This is normally done by identification of a common measure of effect size, which is modelled using a form of meta regression. Resulting overall averages when controlling for study characteristics can be considered meta effect sizes, which are more powerful estimates of the true effect size than those derived in a single study under a given single set of assumptions and conditions.
The first meta-analysis was performed by Karl Pearson in 1904, in an attempt to overcome the problem of reduced statistical power in studies with small sample sizes; analyzing the results from a group of studies can allow more accurate data analysis. Although meta-analysis is widely used in epidemiology and evidence-based medicine today, a meta-analysis of a medical treatment was not published until 1955. In the 1970s, more sophisticated analytical techniques were introduced in educational research, starting with the work of Gene V. Glass, Frank L. Schmidt and John E. Hunter. The online Oxford English Dictionary lists the first usage of the term in the statistical sense as 1976 by Glass. The statistical theory surrounding meta-analysis was greatly advanced by the work of Nambury S. Raju, Larry V. Hedges, Harris Cooper, Ingram Olkin, John E. Hunter, Jacob Cohen, Thomas C. Chalmers, and Frank L. Schmidt.
Contents |
[edit] Advantages of Meta analysis
Advantages of Meta-Analysis (e.. over classical literature reviews, simple overall means of effect sized etc.) include:
- Deriving and statistical testing of overall factors / effect size parameters in related studies
- Generalization to the population of studies
- Ability to control for between study variation
- Including moderators to explain variation
- Higher statistical power to detect an effect than in ‘n=1 sized study sample’
[edit] Steps in a meta analysis
1. Selection of studies (‘incorporation criteria’)
- Based on quality criteria, e.g. the requirement of randomization and blinding in a clinical trial
- Selection of specific studies on a well-specified subject, e.g. the treatment of breast cancer.
2. Search of Literature
- Decide whether unpublished studies are included to avoid publication bias (file drawer problem: see below)
3. Decide which dependent variables or summary measures are allowed. For instance:
- Differences (discrete data)
- Means (continuous data)
- Hedges g is a popular summary measure for continuous data that is standardized in order to eliminate scale differences, but it incorporates an index of variation between groups:
in which μt is the treatment mean, μc is the control mean, σ2 the pooled variance.
4. Model selection (see next paragraph)
[edit] Meta regression models
Generally, three types of models can be distinguished in the literature on meta analysis: simple regression, fixed effects meta regression and random effects meta regression.
[edit] Simple Regression
The model can be specified as
Where yj is the effect size in study j and β0(intercept) the estimated overall effect size.  are parameters specifying different study characteristics.
are parameters specifying different study characteristics.  specifies the between study variation. Note that this model does not allow to specify within study variation.
specifies the between study variation. Note that this model does not allow to specify within study variation.
[edit] Fixed effects meta Regression
Fixed effects meta regression assumes that the true effect size θ is normally distributed with  where
where  is the within study variance of the effect size. A fixed effects meta regression model thus allows for within study variability, but no between study variability because all studies have expected fixed effect size θ, i.e.
is the within study variance of the effect size. A fixed effects meta regression model thus allows for within study variability, but no between study variability because all studies have expected fixed effect size θ, i.e.  .
.
Where 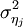 is the variance of the effect size in study j. Fixed effects meta regression ignores between study variation. As a result, parameter estimates are biased if between study variation can not be ignored. Furthermore, generalizations to the population are not possible.
is the variance of the effect size in study j. Fixed effects meta regression ignores between study variation. As a result, parameter estimates are biased if between study variation can not be ignored. Furthermore, generalizations to the population are not possible.
[edit] Random effect meta regression
Random effect meta regression rests on the assumption that θ in  is a random variable following a (hyper-)distribution
is a random variable following a (hyper-)distribution 
Where again  is the variance of the effect size in study j. Between study variance
is the variance of the effect size in study j. Between study variance  is estimated using common estimation procedures for random effects models (restricted maximum likelihood (REML) estimators).
is estimated using common estimation procedures for random effects models (restricted maximum likelihood (REML) estimators).
[edit] Weaknesses
A weakness of the method is that sources of bias are not controlled by the method. A good meta-analysis of badly designed studies will still result in bad statistics. Robert Slavin has argued that only methodologically sound studies should be included in a meta-analysis, a practice he calls 'best evidence meta-analysis'. Other meta-analysts would include weaker studies, and add a study-level predictor variable that reflects the methodological quality of the studies to examine the effect of study quality on the effect size. Another weakness of the method is the heavy reliance on published studies, which may increase the effect as it is very hard to publish studies that show no significant results. This publication bias or "file-drawer effect" (where non-significant studies end up in the desk drawer instead of in the public domain) should be seriously considered when interpreting the outcomes of a meta-analysis. Because of the risk of publication bias, many meta-analyses now include a "failsafe N" statistic that calculates the number of studies with null results that would need to be added to the meta-analysis in order for an effect to no longer be reliable.
[edit] File drawer problem
The file drawer problem describes the often observed fact that only results with significant parameters are published in academic journals. As a results the distribution of effect sizes are biased, skew or completely cut off. This can be visualized with a funnel plot which is a scatter plot of sample size and effect sizes. There are several procedures available to correct for the file drawer problem, once identified, such as simulating the cut off part of the distribution of study effects.
[edit] Applications in modern science
Modern meta-analysis does more than just combine the effect sizes of a set of studies. It can test if the studies' outcomes show more variation than the variation that is expected because of sampling different research participants. If that is the case, study characteristics such as measurement instrument used, population sampled, or aspects of the studies' design are coded. These characteristics are then used as predictor variables to analyze the excess variation in the effect sizes. Some methodological weaknesses in studies can be corrected statistically. For example, it is possible to correct effect sizes or correlations for the downward bias due to measurement error or restriction on score ranges.
Meta analysis leads to a shift of emphasis from single studies to multiple studies. It emphasizes the practical importance of the effect size instead of the statistical significance of individual studies. This shift in thinking has been termed Metaanalytic thinking. The results of a meta-analysis are often shown in a forest plot.
Results from studies are combined using different approaches. One approach frequently used in meta-analysis in health care research is termed 'inverse variance method'. The average effect size across all studies is computed as a weighted mean, whereby the weights are equal to the inverse variance of each studies' effect estimator. Larger studies and studies with less random variation are given greater weight than smaller studies. Other common approaches include the Mantel Haenszel method and the Peto method. A free Excel-based calculator to perform Mantel Haenszel analysis is available at : http://www.pitt.edu/~super1/lecture/lec1171/014.htm. They also have a free Excel-based Peto method calculator at : http://www.pitt.edu/~super1/lecture/lec1171/015.htm
A recent approach to studying the influence that weighting schemes can have on results has been proposed through the construct of gravity, which is a special case of combinatorial meta analysis.
[edit] References
- Cooper, H. & Hedges, L.V. (1994). The Handbook of Research Synthesis. New York: Russell Sage.
- Cornell, J. E. & Mulrow, C. D. (1999). Meta-analysis. In: H. J. Adèr & G. J. Mellenbergh (Eds). Research Methodology in the social, behavioral and life sciences (pp. 285--323). London: Sage.
- Norman, S.-L. T. (1999). Tutorial in Biostatistics. Meta-Analysis: Formulating, Evaluating, Combining, and Reporting. Statistics in Medicine, 18, 321-359.
- Sutton, A.J., Jones, D.R., Abrams, K.R., Sheldon, T.A., & Song, F. (2000). Methods for Meta-analysis in Medical Research]. London: John Wiley. ISBN 0-471-49066-0 [http://www2.le.ac.uk/departments/health-sciences/extranet/research-groups/biostatistics/ajs22/meta/book.html
[edit] See also
- Epidemiologic methods
- Meta-analytic thinking
- Newcastle–Ottawa scale
- Review journal
- Study heterogeneity
- Systematic review
[edit] External links
- Cochrane Handbook for Systematic Reviews of Interventions
- Effect Size and Meta-Analysis (ERIC Digest)
- Meta-Analysis in Educational Research (ERIC Digest)
- Meta-Analysis: Methods of Accumulating Results Across Research Domains (article by Larry Lyons)
- Meta-analysis (Psychwiki.com article)
[edit] Software
- ClinTools (commercial)
- Comprehensive Meta-Analysis (commercial)
- MIX Excel-based tool for meta-analysis (free)
- What meta-analysis features are available in Stata? (free add-ons to commercial package)
- The Meta-Analysis Calculator free on-line tool for conducting a meta-analysis
- Metastat (Free)






